March 2020
Cytologic Evaluation of Peripheral Lymph Node Aspirates
By Dr. Brandy Kastl
Cytologic evaluation of fine needle aspirates from easily accessible enlarged peripheral lymph nodes is a minimally invasive initial diagnostic step in the evaluation of lymphadenomegaly. This article will provide an introduction to differentiating reactive lymphoid hyperplasia from large cell lymphoma in peripheral lymph node aspirates. Readers are encouraged to watch this video produced by KSVDL for guidance on fine needle aspiration and slide preparation, https://www.youtube.com/watch?v=JTYJBNxeTH8. High cell recovery during aspiration followed with a well-prepared cytologic smear makes the difference between the joy of an accurate cytologic diagnosis and the frustration of a non-diagnostic sample.
Step 1: Knowing when and where to sample
As with aspiration technique and slide preparation, an accurate cytologic diagnosis may also depend on which nodes are selected for sampling and when those nodes are sampled in the disease course. For instance, mandibular lymph nodes are often responding to antigenic stimulation from the oral cavity which may hinder accurate differentiation between a reactive and neoplastic lymphoid population. If multiple lymph nodes are enlarged, avoid sampling only the mandibular lymph nodes as they may not be fully representative of all affected nodes. Obtaining at least two to three aspirates from each enlarged lymph node is best. All cytologic samples should be taken prior to administering medications, particularly gluccocorticoids and antimicrobials. In the case of lymphoma, gluccocorticoids induce death of neoplastic lymphocytes reducing their numbers and potentially resulting in a misinterpretation of cytologic findings. Antimicrobials can reduce infectious organisms making it hard or impossible to identify them in cytologic preparations. Additional tips for improving the diagnostic accuracy of your lymph node aspirates are provided in the table below, Lymph Node Cytology Pro Tips.
| Lymph Node Cytology Pro Tips |
|
Step 2: Determining if your sample is diagnostic
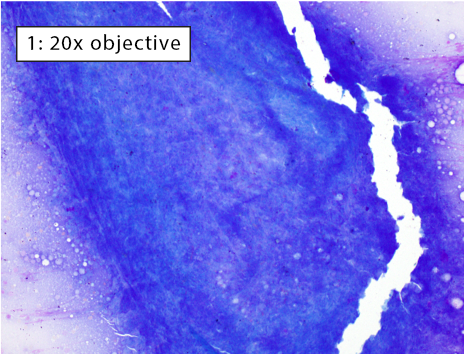
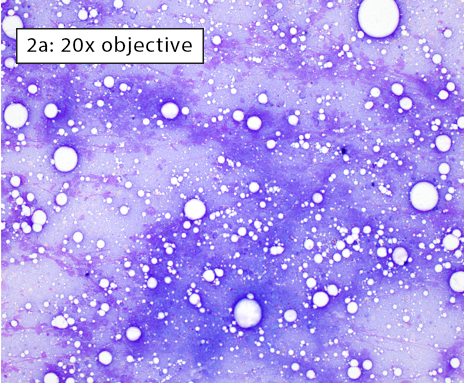
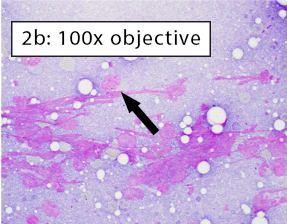
After collecting a cytologic sample, the next step is to determine if the preparation adequately represents the aspirated tissue and will yield an accurate cytologic diagnosis. This is determined by answering these questions: 1) Are enough cells present?; 2) Are the cells well spread?; and 3) Are the well-spread cells intact? The three images below depict different areas of the exact same slide which we will use to answer answer the three questions above.
|
Figure 1: Non-diagnostic due to lack of well-spread, intact cells Many cells are present, but most cells are thickly piled preventing adequate evaluation. A few intact cells are visible near the edges of the image. However, there are too few intact, well-spread cells to be considered fully representative of the entire lymphoid population within an enlarged lymph node. Thick cell aggregates most commonly occur when aspirates are not smeared due to fear of cell rupture or when using a needle tip to spread the cells (i.e. starfish preparations). |
|
Figure 2a-b: Non-diagnostic due to a lack of intact cells Cells have been spread but are no longer intact. Long, thin, purple streaks of chromatin, known as nuclear streaming, are visible along a line parallel to the spreading direction. In figure 2b, some rounded nuclei are still apparent (black arrow). However, these cells lack both distinct, crisp cytoplasmic and nuclear membrane borders indicating they are no longer intact. Nuclear streaming most often occurs when excessive downward pressure is used during smearing. It can also happen when smearing does not occur immediately and cell droplets dry on the slide. |
|
|
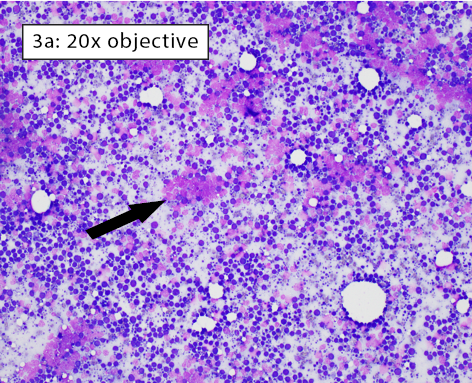
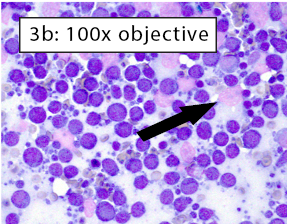
Figure 3a-b: Area of excellent diagnostic quality Cells are well-spread and many intact cells are present. Well-spread cells have some distance between them allowing for easy visualization of individual cell features. Large lakes of amorphous purple chromatin are still present (black arrow). However, the majority of cells are intact. With higher magnification in Figure 3b, the majority of cells have crisp, distinct cytoplasmic and nuclear membranes. Some ruptured cells are still present (black arrow). |
|
Step 3: Knowing how to size lymphocytes
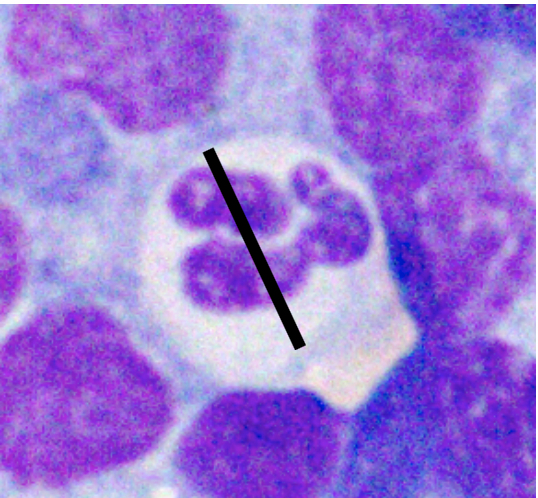
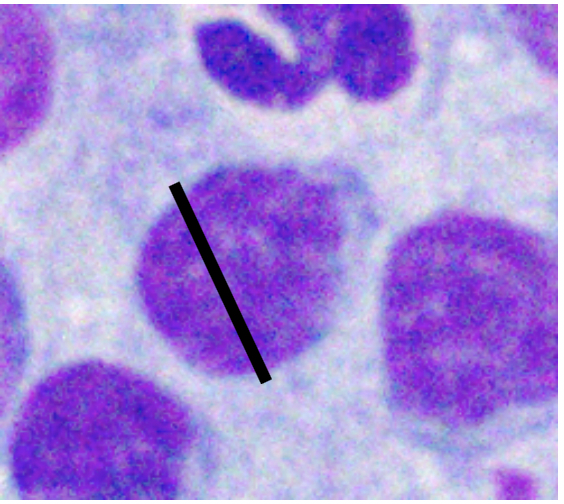
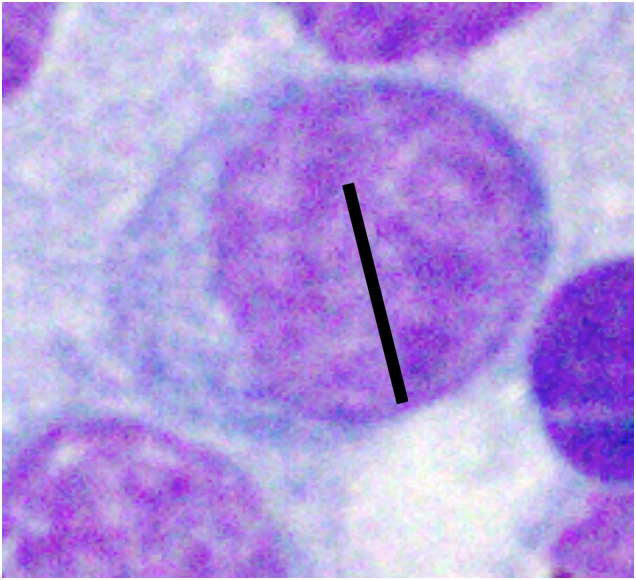
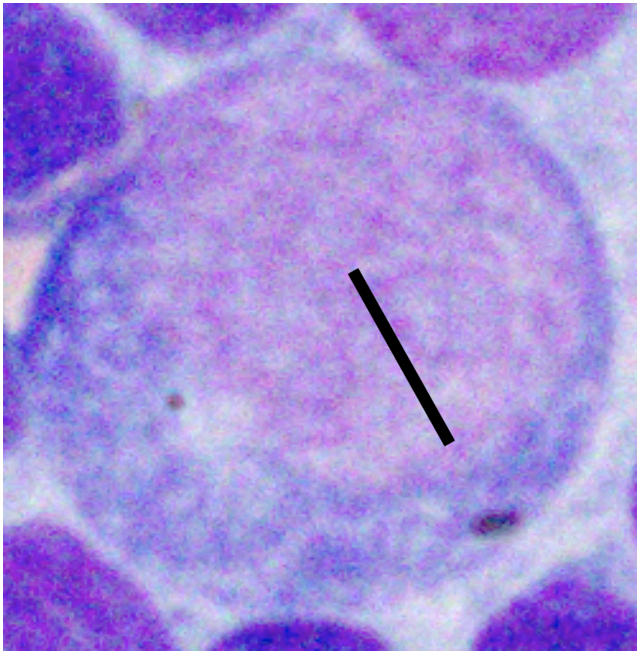
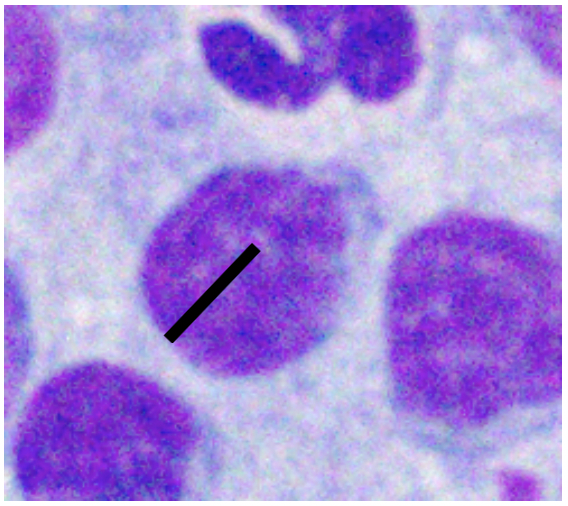
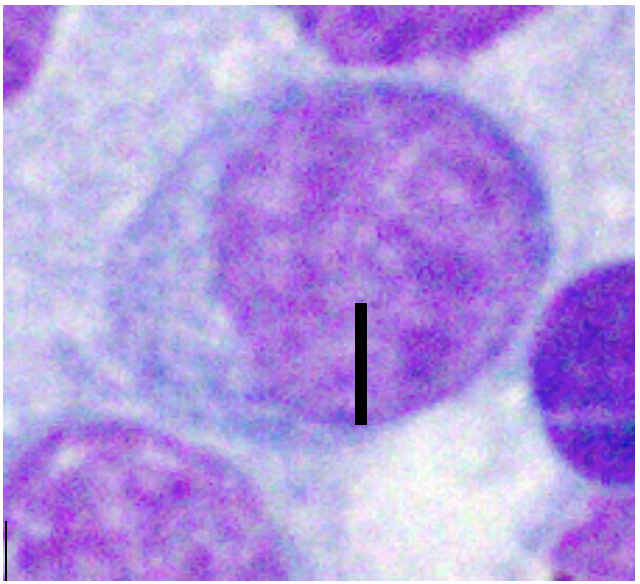
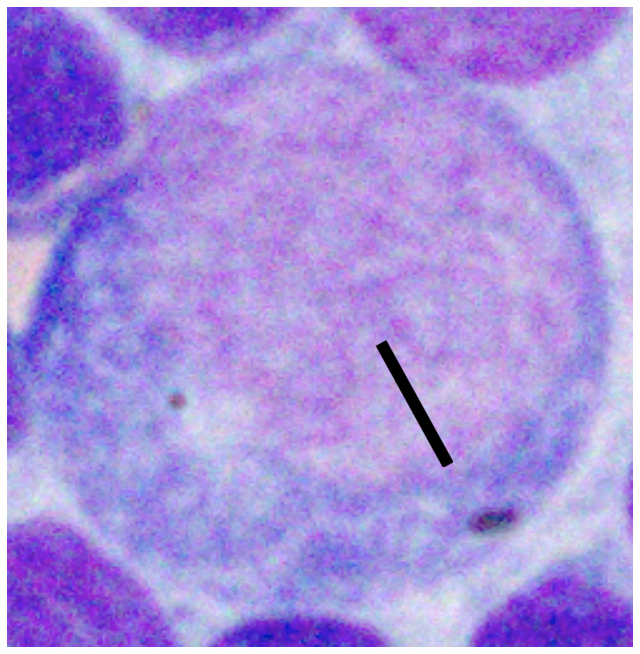
Various factors can affect how lymphocytes appear both within a single cytologic sample from one lymph node and between samples from different nodes. For instance, enlarged lymph nodes may be edematous which can cause lymphocytes to appear condensed and balled-up within a light blue background. Correctly determining the size of lymphocytes is critical to achieving an accurate diagnosis. In dogs, lymphocytes can be sized by comparing the diameter of the lymphocyte nucleus to either an intact neutrophil or an erythrocyte. The table below summarizes lymphocyte sizing compared to each of these cell types. Importantly, these general lymphocyte sizing guidelines apply to canine lymphocytes only.
| How to Size Canine Lymphocytes | |||
| Small Lymphocyte | Medium Lymphocyte | Large Lymphocyte | |
| Intact Neutrophil Diameter | Nucleus < 1.0x | Nucleus 1.0-2.0x | Nucleus >2.0 x |
|
|
|
|
| Well-spread RBC Diameter | Nucleus 1.0-1.5x | Nucleus 2-2.5x | Nucleus ≥ 3.0 x |
| |
|
|
|
Step 4: Differentiating between homogeneous and heterogeneous lymphoid populations
Unlike cytologic evaluation of other tissues, some variation in cell size (anisocytosis) and nuclear size (anisokaryosis) is expected in a normal healthy lymphoid population. This variety in lymphoid appearance is due to the normal variety in lymphoid cells necessary for an appropriate immune response. After identifying a well-spread area of intact cells, assess the lymphoid population as a whole at low magnification such as with a 10x, 20x, or 40x dry objective. In general, heterogeneous lymphoid populations are reactive and homogeneous lymphoid populations are neoplastic.
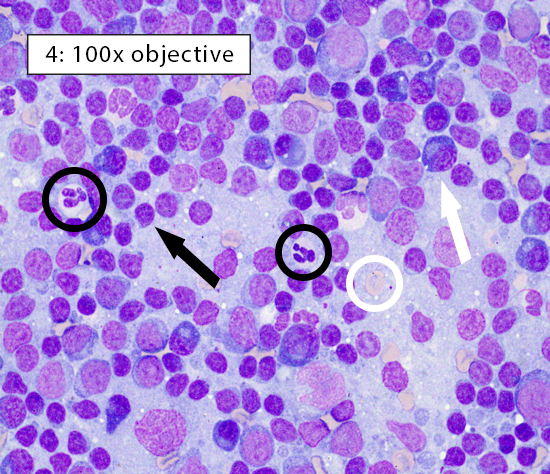
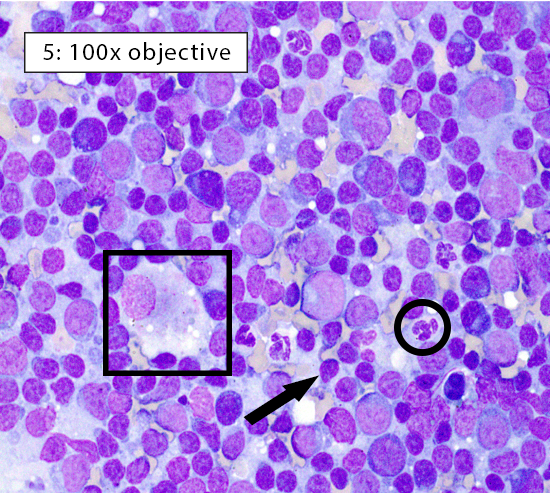
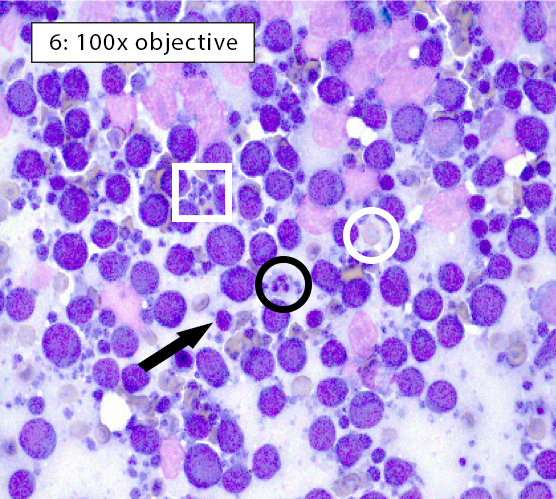
Image key: Black circle = intact neutrophils, White circle = well-spread erythrocyte; Black arrow = normal small lymphocyte; White arrow = plasma cell; Black box = histiocyte (ie dendritic cell); White box = cytoplasmic fragments
|
Figure 4: Reactive lymphoid hyperplasia In heterogeneous lymphocyte populations, small lymphocytes comprise ~60-70% of the lymphoid cell population. The remaining cells are a mixture of medium lymphocytes, large lymphocytes, plasma cells, histiocytes/dendritic cells, and other inflammatory cell populations. Lymphocytes here are heterogeneous and predominately small (black arrow) with frequent plasma cells (white arrow) consistent with reactive lymphoid hyperplasia. |
|
Figure 5: Reactive lymphoid hyperplasia Reactive lymphoid hyperplasia can occur in response to B-cell activation, increased numbers of antigen presenting cells, or both. In this heterogeneous population of lymphocytes, small lymphocytes (black arrow) still comprise ~60-70% of the total population. Many plasma cells (white arrow) are present indicating B-cell activation within the lymph node. In addition, the large histiocyte (black box) indicates increased numbers of antigen presenting cells. Histiocytes (ie dendritic cells) can be differentiated from large lymphocytes by their abundant pale gray-blue cytoplasm and often indistinct cellular borders. |
|
Figure 6: Medium cell lymphoma In homogeneous lymphocyte populations, ≥70-80% of the lymphoid population is comprised of lymphocytes of a single size, usually medium or large. Homogeneous lymphocytes will also display similarity in nuclear and cytoplasmic features. In this case, the majority lymphocytes are medium in size (nuclei ~1.0-2.0x a neutrophil) with few small lymphocytes present. Many small blue fragments of cytoplasm, aka lymphoglandular bodies (white box), are often scattered throughout the background in cases of lymphoma. They can be quite large and mimic intact cells or platelet clumps, but these cytoplasmic fragments do not have nuclei. It’s always important verify that the structures you are interpreting as cells have visible nuclei. Otherwise, this could be misdiagnosed as a reactive lymphoid population. |
Step 5: Getting a pathologist consultation
It’s important to remember that fine needle aspirates are only a small sampling of the entire lymphoid population within a lymph node. There are instances when a cytologic diagnosis is not clear cut. For instance, predominant lymphocyte cell size may vary from slide to slide within the same node. This usually happens when a reactive lymph node has large germinal centers filled with abundant large lymphocytes or when a neoplastic lymph node has not yet been completely effaced by neoplastic lymphocytes. It’s best to assume that every lymph node aspiration will eventually require a clinical pathologist’s opinion. Following the tips provided in the table entitled “Lymph Node Cytology Pro Tips” can help improve the diagnostic quality of lymph node aspirates and ensure that a cytologic diagnosis can be achieved. Stay tuned for the next article in this series on peripheral lymph node cytology. We’ll be addressing steps you can take when a lymph node sample of excellent diagnostic quality fails to yield a definitive cytologic diagnosis.
Brandy Kastl, DVM, DACVP is a Clinical Pathologist in the Kansas State Veterinary Diagnostic Laboratory