
October 2023
Specimen Collection for Diagnostic Testing: The Quality of Diagnostic Samples
By Hatem Kittana, DVM, PhD, DACVM
Proper specimen collection is the most important step in the recovery of the etiologic agent in infectious disease diagnosis. Improperly or poorly collected specimens may lead to the failure to isolate the causative agent and/or results in the recovery of contaminants, which can lead to an incorrect diagnosis and therapy.
 |
 |
|
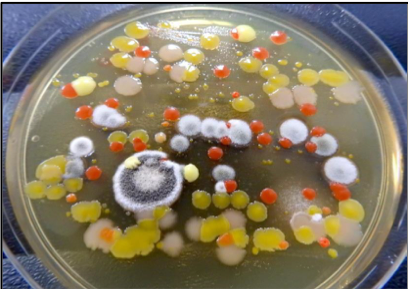
Results obtained from veterinary clinical samples are a direct reflection of the quality of the specimen that was submitted: Garbage in – garbage out! Left: picture A; right: picture B |
|
Picture A: a heavily contaminated placenta sample was submitted for bacterial culture “abortion panel screen”
Picture B: a culture plate of placenta sample in picture A after 24 hours incubation showing heavy mixtures of non-significant bacterial and fungal growth (e.g., environmental contaminants)
There are important factors that should always be considered when selecting a specimen for bacterial or fungal culture:
- Origin of specimen material:
The sample should be representative of the actual site of infection with a minimal amount of contamination from the surrounding areas. Clip and scrub the sampling sites as appropriate. Take deep samples rather than surface samples from wounds and draining tracts, and aspirate closed lesions rather than sampling draining lesions when possible. Sampling from the edges of a lesion is important, as that is where the most actively growing organisms will be found.
- Optimal time for specimen collection:
The optimal collection time for recovery of the etiologic agent varies according to the nature of the infectious disease. It is important to collect specimens from an animal that is acutely affected rather than chronically affected. When culturing a chronically affected animal, secondary infections may mask the presence of the primary agent. In deceased animals, anaerobic overgrowth from the GI tract occurs within 4 hours of death. Anaerobic samples must be taken as soon after death as possible.
- Quantity of specimen:
A sufficient volume of material must be obtained to perform the culture techniques requested. One swab is usually not a sufficient sample for a full-set of cultures (e.g., bacterial, fungal, viral). Even if there is not sufficient sample to collect measurable fluid, you can always send more than one swab from the same lesion. For tissue samples, send at least 2 inches of fresh tissue for culture and do not forget to submit fetal stomach contents and placenta (with cotyledons) in abortion cases!
- Collection prior to drug treatment:
Whenever possible, collection should precede the administration of antimicrobials. If this is not possible, the causative agent may still be recovered because many antibiotics are bacteriostatic, not bactericidal.
- Proper labeling:
Ensure that your sample is properly labeled in a way that will not be smeared. Please make sure both the submission form and sample labeling include animal ID, site of sampling and date obtained. Label the container portion of containers with lids and not the lid (lids can be mixed up between containers.) Place the submission form in a separate Ziploc bag taped to the sample container; this way if the sample leaks the papers will still be legible.
- Storage and shipment:
Tissue samples should be sealed into a screw-top vial, Ziploc bag or Whirl-Pak bag. Small tissue samples at risk of desiccation may be wrapped in sterile saline-soaked gauze. Samples that cannot be immediately shipped should be stored at refrigerator temperature (do not freeze! – only exception is milk samples). The exception are anaerobic samples: anaerobic samples should be stored/shipped at room temperature. Ship by the most expeditious means possible and ensure temperature control during shipment with cold packs and insulated boxes. All sample containers should be sealed within a second leak-proof container (e.g. Ziploc bag or Whirl-Pak) that contains absorbent material in the event of leaks. Shipping companies will refuse or discard leaky packages.
Finally, if unsure concerning sample collection or handling, please call the KSVDL bacteriology lab at 785-532-4468 for advice. We strive to assist our fellow veterinarians and clients on the best sampling, storage, and transport methods for their sample so that chances of a meaningful diagnostic result are maximized.
Hatem Kittana, DVM, PhD, DACVM is the Assistant Professor of Veterinary Microbiology, Bacteriology/Mycology Section Head, Kansas State Veterinary Diagnostic Laboratory (KSVDL)